Testing to dynamic, in-use conditions for safer handling of chemotherapy drugs
As the global incidences of cancer rise, so will the need to ensure the safe handling of chemotherapy drugs in your workplace.
Ansell gloves are tested to EN 16523-1 and ASTM D6978 standards3, or both, to ensure gloves meet the requirements for safe handling of hazardous drugs and their intended use either as medical devices or personal protective equipment (PPE). But we don’t stop there.
Ansell’s Cytostatic Permeation Program (ACPP), a unique dynamic permeation test, has been designed to give you an added in-use perspective of permeation detection to help you select the right glove for safer handling of chemotherapy drugs.
Working conditions are dynamic, so why not test to in-use conditions?
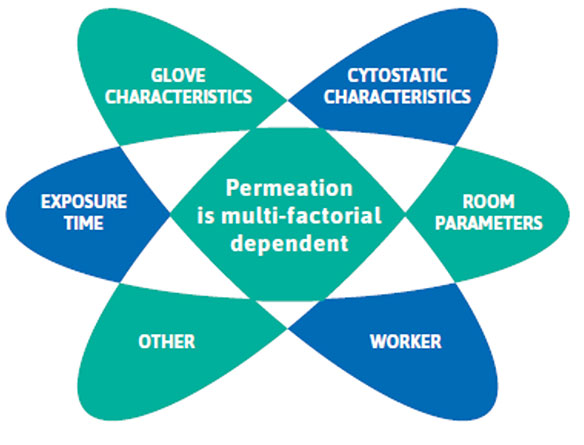
While ASTM and EN standards determine glove permeation under static test conditions, ACPP mimics the everyday use in your workplace through controlled dynamic conditions.
Current standards testing does not consider all working conditions that may influence the permeation of your glove’s protective barrier. Such influencing factors are for example the concentration and exposure time of the chemotherapy drug being handled, glove properties such as thickness; the stretching and flexing motion of the task, as well as both body and workplace temperatures.4, 5, 6, 7
It is important you consider how static (standards) vs dynamic (ACPP) testing differs to gain a comprehensive view of how the permeability of a glove to a chemotherapy drug is affected under the different conditions.
Maximize your confidence by minimizing your exposure
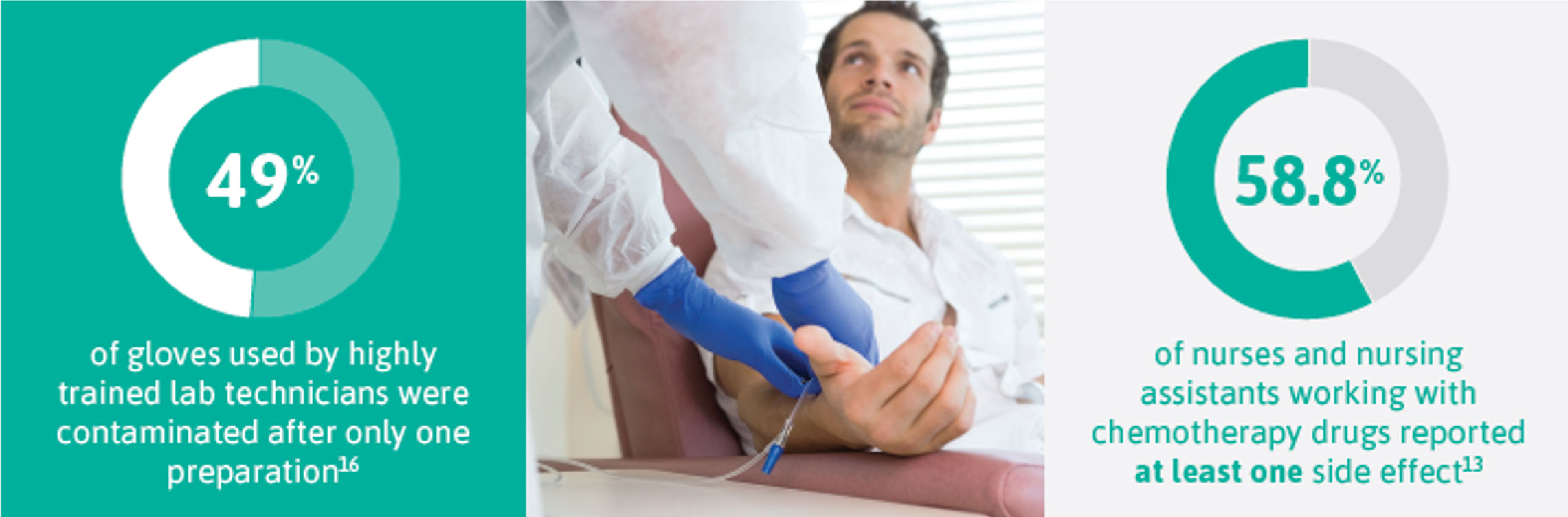
There is no safe level of exposure to chemotherapy drugs.9, 10 Some chemotherapy drugs are more toxic than others. The earlier the permeation of a hazardous drug is detected, the safer your risk assessment becomes.
Minimizing the risks of exposure starts with using the available data that best guides you by presenting the earliest possible point of permeation detection.
EN and ASTM detection limits, based on the permeation rate of 1.0 and 0.01μg/cm²/min respectively, are the defined thresholds applicable to all drugs tested to these standards.
ACPP however, detects for permeation of each drug at its lowest limit of detection which, depending on the chemotherapy drug, varies from 0.00011 to 0.000001μg/cm²/min. This ability for earlier detection is made possible by the highly sensitive analytical devices used.11
Choosing the right glove: What you need to know
Unwanted side effects occur not only in cancer patients undergoing treatment but pose risks for healthcare workers who handle chemotherapy drugs.
Reported effects range from headaches, irritation of eyes/skin, hair loss and dizziness to adverse outcomes including genetic damage leading to infertility, cancer and miscarriages. 13, 14, 15
Whether you are reconstituting or administering chemotherapy treatments or involved in clean-up and disposal, the right, chemotherapy-tested gloves must be in place because the primary route of occupational exposure is your skin, directly or indirectly.
The largest group exposed is often pharmacy staff involved in drug preparation due to the frequency of use, and the quantities and concentration used.17
References: 1. IARC, GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. 2. IARC GLOBOCAN 2018, Global Fact Sheet, accessed 13 September, 2020: https://www.uicc.org/news/new-global-cancer-data-globocan-2018. 3. ASTM is the American Society of Testing & Materials; and EN is the European Norm. 4. Nalin M, Hug G, Boeckmans E, Machon C, Favier B and Guitton J. Permeation measurement of 27 chemotherapy drugs after simulated dynamic testing on 15 surgical and examination gloves: A knowledge update. Journal of Oncology Pharmacy Practice. 2020;0(0):1-14. 5. Landeck L, Gonzalez E, Koch OM. Handling chemotherapy drugs-Do medical gloves really protect?. Int J Cancer. 2015;137(8):1800-1805. doi:10.1002/ijc.29058. 6. Phalen RN, Le T, Wong WK. Changes in chemical permeation of disposable latex, nitrile, and vinyl gloves exposed to simulated movement. J Occup Environ Hyg. 2014;11(11):716-721. doi:10.1080/15459624.2014.908259. 7. Dillon J and Schroeder L. Permeability and material characteristics of vulcanized latex film during and following cyclic fatigue in a saline environment. Journal of Applied Polymer Science, 1997;64(3):553-566. 8. At the time of publishing, Ansell is the only glove manufacturer with its own dynamic testing device called the Ansell Dynamic Testing Device (ADTD). 9. Sessink PJ, Bos RP. Drugs hazardous to healthcare workers. Evaluation of methods for monitoring occupational exposure to chemotherapeutic drugs. Drug Saf. 1999;20:347–59. 10. Oriyama T, Yamamoto T, Yanagihara Y, et al. Evaluation of the permeation of antineoplastic agents through medical gloves of varying materials and thickness and with varying surface treatments. J Pharm Health Care Sci. 2017;3(13). 11. ACPP uses Inductively coupled plasma-mass spectroscopy (HPLC-DAD) and liquid chromatography-mass spectrometry (LC-MS/MS). 12. Cancer Medicine 4th Edition, Encyclopedia of Cancer, Cancerologie Clinique Thérapeutique du Cancer, Compendium 20th Edition. 13. Ivanova K, Avota M. Antineoplastic Drugs: Occupational Exposure and Side Effects. Proceedings of the Latvian Academy Of Sciences. 2016;70(5):325–329. doi:10.1515/prolas-2016-0049. 14. Hon C, Teschke, K Demers, P. Venners, S. Antineoplastic drug contamination on the hands of employees working throughout the hospital medication system. Ann Occup Hyg. 2014;58(6): 761-770. 15. Tracy Wyant, DNP, RN-BC, AOCN®, CHPN®, CPPS. https://voice.ons.org/newsand-views/what-is-onss-stance-on-handling-chemotherapy-while-pregnant-breastfeeding-or-trying. Accessed 17 Sept 2020. 16. Bertrand Favier Thesis, Hospital Pharmacist, Centre Régional Léon-Bérard, Lyon – France. 17. Hall A, Demers P, Astrakianakis G, Ge C, and Peters C. Estimating National-Level Exposure to Antineoplastic Agents in the Workplace: CAREX Canada Findings and Future Research Needs. Annals of Work Exposures and Health. 2017;61(6):656-658. 18. https://www.usp.org/compounding/general-chapter-hazardous-drugs-handling-healthcare. Accessed 17 Sept. 2020.