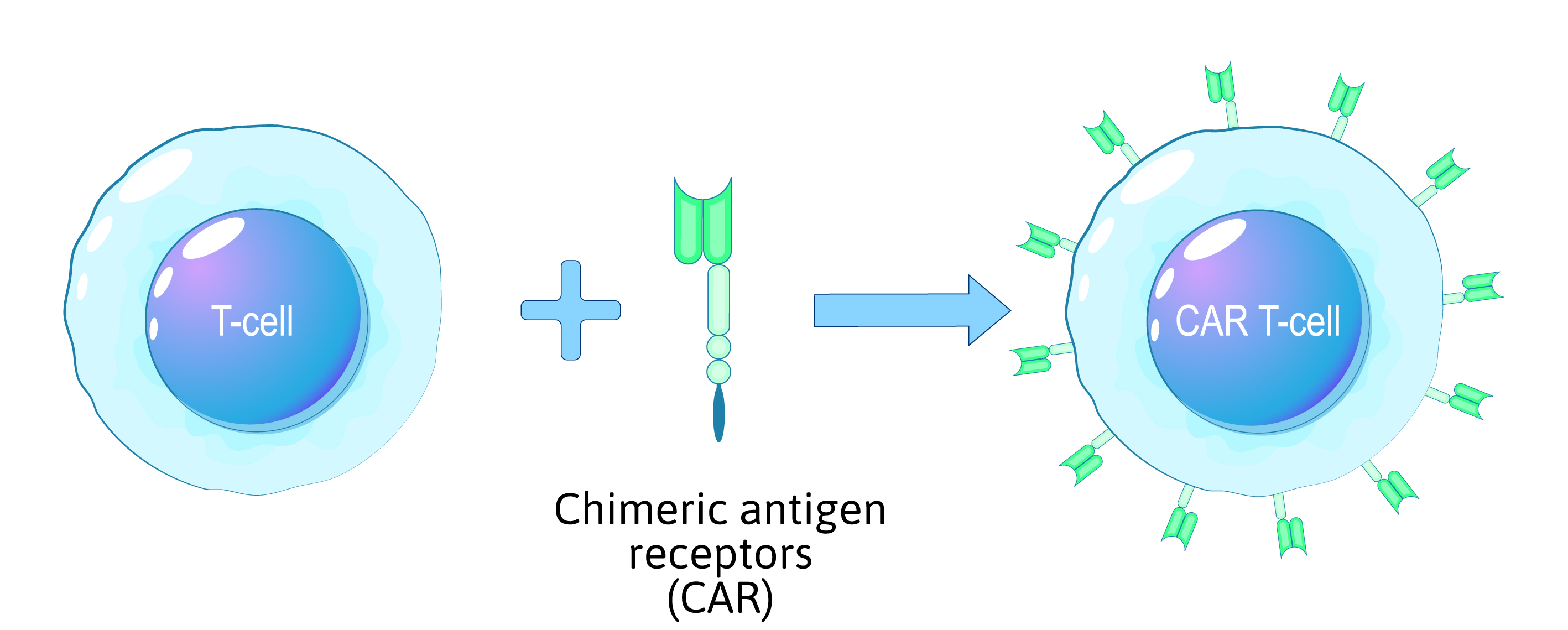
CAR T-cell therapy, an innovative approach to treating certain types of cancer, relies on the genetic modification of a patient's immune cells to target and destroy cancer cells. This complex process requires highly specialized laboratory procedures, and it is essential to prioritize the safety of the modified cells, the patient, and the healthcare professionals involved.
This article delves into the CAR T-cell genetic modification process, the crucial role contamination control plays, and the critical role of Personal Protective Equipment (PPE) in maintaining a secure environment.
The CAR T-Cell Genetic Modification Process
-
Collection of T Cells:
- The CAR T-cell therapy process begins with the collection of a patient's T cells through leukapheresis, a procedure similar to blood donation.
- Leukapheresis separates T cells from other blood components, ensuring a high concentration of the target cells.
- Ensuring a clean and sterile collection environment is essential to prevent contamination, as any compromise at the start of the process can impact the effectiveness of the therapy.
-
Isolation and Activation:
- Once collected, the T cells are isolated and activated in the laboratory. They are then cultured to ensure they are in an active state and ready for modification.
- Once collected, the T cells are isolated and activated in the laboratory. They are then cultured to ensure they are in an active state and ready for modification.
-
Genetic Modification:
- Genetic modification is a critical step in the CAR T-cell process. Scientists introduce a new genetic sequence into the T cells to enable them to express the Chimeric Antigen Receptor (CAR).
- This CAR is engineered to recognize specific antigens found on the surface of cancer cells.
- Lentiviral or retroviral vectors are typically used to deliver the CAR gene into the T cells' genome. Strict contamination control ensures the integrity of the genetic modification process.
-
Expansion:
- After genetic modification, the CAR T cells are expanded in culture to create a larger population of these specialized immune cells. This step ensures there are enough CAR T cells to mount an effective immune response.
Figure 1: Overview of the CAR T-cell reprogramming process using chimeric antigen receptors (CAR).
- After genetic modification, the CAR T cells are expanded in culture to create a larger population of these specialized immune cells. This step ensures there are enough CAR T cells to mount an effective immune response.
-
Quality Control:
- Rigorous quality control measures are in place to ensure that the modified T cells are safe and effective. This includes assessing the purity, viability, and functionality of the CAR T cells.
- Rigorous quality control measures are in place to ensure that the modified T cells are safe and effective. This includes assessing the purity, viability, and functionality of the CAR T cells.
-
Infusion into the Patient:
- The final step involves infusing the modified CAR T cells back into the patient's bloodstream. This infusion marks the beginning of the therapeutic phase, where CAR T cells recognize and attack cancer cells with the targeted antigen.
- The final step involves infusing the modified CAR T cells back into the patient's bloodstream. This infusion marks the beginning of the therapeutic phase, where CAR T cells recognize and attack cancer cells with the targeted antigen.
Contamination Control Measures
Effective contamination control is imperative throughout the CAR T-cell genetic modification process to maintain the integrity of the therapy and ensure the safety of both patients and laboratory personnel. Key contamination control measures include:
-
Sterile Techniques: Laboratory personnel must strictly adhere to sterile techniques, including handwashing, gowning, and gloving, to ensure that all equipment and materials used are sterile.
-
Biological Safety Cabinets (BSCs): Genetic modification of cells takes place within Class II Biological Safety Cabinets, which provide a controlled and sterile environment. These cabinets help contain potential contaminants and protect laboratory workers.
-
Cleanroom Facilities: CAR T-cell production often occurs in ISO-class cleanroom facilities, which are specifically designed to maintain high levels of cleanliness and control particulate contamination.
-
Isolation Barriers: Physical isolation barriers, such as laminar flow hoods and closed systems, are used to prevent contamination during cell manipulation and culture processes.
-
Routine Monitoring: Regular monitoring of laboratory environments for air quality, surface contamination, and microbial presence is essential to identify and address potential sources of contamination promptly. Common monitoring tools include particle counters and microbial samplers.
-
Waste Disposal: Proper disposal of waste materials, including potentially contaminated equipment and biological waste, is crucial to prevent contamination. The disposal of contaminated materials, including sharps, is vital to prevent any accidental exposure to personnel.